Disney for docs: CAMLS leads training in biotech, medical devices
CAMLS, an innovative medical simulation center in downtown Tampa, is one of the world’s largest free-standing centers fully dedicated to training healthcare professionals.
Most of us who live and work outside of the healthcare industry are mostly unaware of the processes and intricately detailed work that goes into the creation and design of devices used to keep us healthy.
But physicians, engineers, clinical studies professionals, medical directors and product manufacturers who work behind the scenes on the devices go through time-consuming and meticulous processes in medical device development.
Research, creation, design, FDA approval, prototyping and eventual unveiling and release of each medical device add up to quite a tremendous endeavor. Efforts can often meet with roadblocks such as approval delays, costly mistakes or time-consuming searches for the right research team.
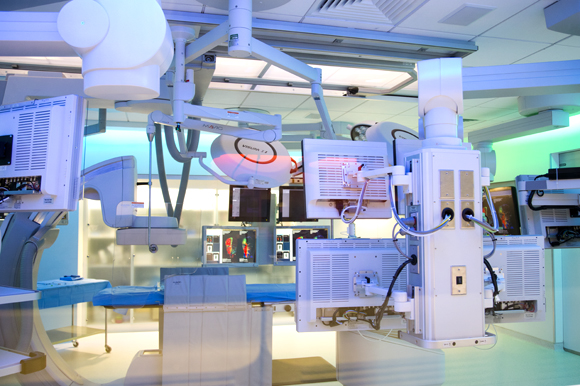
A gorgeous facility in downtown Tampa, a crisp, futuristic Innovation Center — part of the University of South Florida Center for Advanced Learning and Simulation (CAMLS) — is rocking the industry with a brilliantly executed production that streamlines and improves the entire process.
“We get to create incredible things here. We tell people its Disneyland for Doctors,” says Stuart Hart, Chief Medical Officer, innovation and medical education. “And that truly is what its like for doctors here. It is really an exciting place.”
His excitement is infectious, a pretty good indication that what’s going on is cutting edge. Hart is a Fellow of the American College of Obstetrics and Gynecology and the American College of Surgeons. He completed the Leadership Institute Program in the Center for Transformation and Innovation (CTI) at the USF College of Medicine, and the Master of Science Degree in Entrepreneurship in Applied Technologies at the USF College of Business. He also completed the Regulatory Affairs in Medical Devices Certificate Program through the Department of Industrial and Management Systems Engineering within the USF College of Engineering, and is enrolled in the Executive MBA Program at the Massachusetts Institute of Technology.
A description offered up on the Innovation Center’s website indicates it is clearly a great source of pride: As one of the world’s largest free-standing centers fully dedicated to training healthcare professionals, the 90,000-square-foot, three-story facility provides a state-of-the-art, clinical environment with 60,000 square feet dedicated to surgical skills labs, operating suites, a virtual hospital and simulation center, and more than 25,000 square feet of dedicated education and conference space.
“It’s very unique, to bring all those aspects here — and you bring all those significant players within the medical device innovation industry all together,” Hart says. “You also have access to high and low fidelity simulators and laboratories.”
Invention reaches new heights
By providing all the necessary brainpower and expertise, equipment and consultations necessary to take a solution from idea to launch and release, the Innovation Center takes the invention adventure to new heights, says Mark Moyer, an MBA and mechanical engineer who is the newly appointed head of the center.
“Tightened FDA device evaluation and enforcement, the expansion of home health care delivery and the impact of the medical device tax are just a few of the challenges stressing the industry’s ability to move to market quickly,” he says.
Moyer, who received a Bachelor’s Degree in Mechanical Engineering from California State Polytechnic University and an MBA from the University of San Diego, says there is nothing else around like the center.
“The Innovation Center’s location within CAMLS provides unmatched capabilities for our clients. We can speed problem solving and testing, and help deliver the device solutions needed to improve patient safety and outcomes -- all in one high-fidelity facility.”
There’s no dispute about the need for such an operation. It is estimated that 400,000 lives are lost annually due to avoidable medical errors. For more than a decade, analysts and experts have called for innovation to prompt major changes. But obstacles must be overcome, they say. Broad-sweeping, life-altering change calls for insight, and courage. It calls for new ideas and ways of thinking.
In response to that industrywide call for help, the University of South Florida Center for Advanced Medical Learning & Simulation (CAMLS) was created. It opened in February 2012. CEO Deborah Sutherland is credited with recognizing the need for an innovative center where healthcare education and training could be reinvented and practiced in a realistic, clinical learning environment.
It is a great compliment to CAMLS and the Innovation Center that Moyer has joined the group, says Sutherland, who received a PhD from the University of South Florida Tampa, an MSN from Boston University and a BSN from the University of Maine.
“Mark’s attraction to our team speaks to CAMLS’ unique capabilities as a highly desirable business partner,” she says. “His specific expertise in usability studies and compliance are critical to helping clients avoid the expensive mistakes that delay FDA approval and commercialization. “
Moyer brings decades of experience in leadership and training positions across all phases of the medical device life cycle to CAMLS. Touted as one of the industry’s leaders and a sought-after speaker, he is also a certified biomedical auditor.
He came to a modern, showcase facility to work when he took this job. The Innovation Center’s space is 4,200 square feet in a state-of-the art space that offers a prime environment for engineers, sales and marketing teams of clients to collaborate.
“The Innovation Center’s strategic location inside CAMLS provides an unmatched environment for healthcare providers, engineers and academics to collaborate in the design, testing, validation of and training on medical devices,” Moyer says.
“Florida ranks number 2 nationally in its concentration of FDA-registered medical device companies, and because of CAMLS’ full-service capabilities to the industry, it is able to help speed improved medical device solutions to patients and improve outcomes.”
CAMLS is also establishing a presence in Panama. The USF Health International Foundation office is located there, and CAMLS is working with the country of Panama to develop a CAMLS-like center. Central American doctors would be trained in best practices for the specialties they need to improve the delivery of care and have better patient outcomes.
“The overall goals of CAMLS’ Innovation Center are to reduce healthcare costs and improve patient outcomes by being involved in the development of the next game-changing medical devices and combination products,” Moyer says.
Every step of the way
When clients come to CAMLS with an idea for a device – or just the awareness that some solution is needed — every level of the process can be handled at the Innovation Center. “It brings not only those involved with the innovation concept, but industry and health care professionals together at a common site,” says Paul Ayers, Director of Sales, Marketing and Business Development.
Clients do not have to shop around for experts who can assist them at each level of the process in product creation and development once they engage with the Innovation Center, says Ayers, who holds a degree in American Studies from the University of South Florida.
The center offers for the device design cycle — engineering and fabrication — realistic high-fidelity simulation labs and qualified staff to problem-solve. Conceptualization, building and prototyping, testing and validating is all handled on-site.
There’s a 3-D printer, and on-site engineering and testing is provided. At the center, clients can receive design consultation, human factors engineering, engineering and modeling services, usability engineering assessments, regulatory and quality review and design verification and validation. And that’s just to name a few.
Mario Simoes, operations manager and chief engineer, says partnerships, such as those developed with Stryker, MedCure, Inc. Synergy Health, Occammd and Philips, add tremendous strength to the operation. And a major emphasis has been on working with local experts, he says. “We have tried to keep just about everything local.”
The convenience for clients is a draw. And they are relieved of having to concern themselves with rounding up researchers, getting FDA approval and, in many instances, creating the device. “They come up with the dream, and we have to come up with a way to make it come true,” Simoes says.
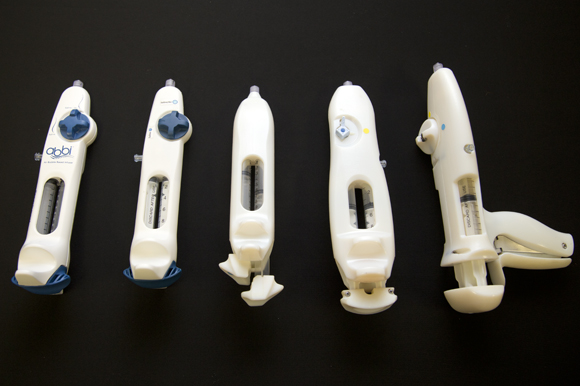
CooperSurgical Inc. (CSI) used the center for several smaller projects before selecting CAMLS as a full partner in the design and development of a new device, released in May.
The product is called ABBI (trademark), which stands for Air Bubble Based Infuser. The company’s Chief Medical Officer, Robert Auerbach, M.D., says it’s “a disposable device intended to aid in the identification of fallopian tube patency and other reproductive system pathologies.”
The experience of creating ABBI at CAMLS was very positive, the company reports. “Working with the team at CAMLS is always invigorating,” says Peter Arneson, senior product manager with CooperSurgical. “Their knowledge, along with a can-do attitude, provided thoughtful solutions to complex clinical and mechanical scenarios. I’ve enjoyed the process and look forward to further joint projects.”
CAMLS, which served more than 30,000 learners since opening in March of 2012, is just getting started.
Those involved say they are excited about the future, and clients are, as well. “It definitely will take off,” Simoes says. Our clients are very happy. Things here go at a much faster pace. They are realizing the capabilities.”
The professionals who make things happen seem to be just as happy to be there. “It is a terrific, terrific place,” Hart says. “Every day it’s something new and exciting.”