Could bacteria eaters save the planet?
No pressure, but: A growing consensus within medical science is that the future of humanity could lie in the phage positioned under students' microscopes at the University of South Florida.
As the end of fall semester looms, a lab at USF buzzes with focused energy. Students in the SEA-PHAGES program are preparing DNA samples for gel electrophoresis, each of them working to isolate a unique bacteriophage — a microscopic organism known colloquially as a “phage,” or by its charming Latin root translation “bacteria eater.”
Phage are highly individualized, innumerably varied, naturally occurring microscopic organisms. They replicate by infecting bacteria with debilitating viruses, but unlike chemical antibiotics, phages are so highly specialized that they attack only specific strains. In other words: phage are very picky bacteria eaters — and that’s a good thing, because they leave “good” bacteria, like human gut flora, alone.
The handful of phage samples whose genomes students merit further study
will travel from Tampa to the University of Pittsburgh for DNA sequencing over winter break. When they return to USF for spring semester, the SEA-PHAGES students will run computer-based genetic analyses on their newly-sequenced phage.
While his students work, SEA-PHAGES USF instructor, Dr. Richard Pollenz, offers a line of Socratic questioning to encourage scientific rigor.
“The first semester is all about getting a wet lab experience and learning microbiology techniques. Students learn math exercises to calculate numbers of phage — and we’re talking about huge numbers: billions and billions of phage in each sample,” Pollenz says.
Moments later, he’ll press his students to tell him exactly how they plan to dilute their samples enough to isolate an individual, microscopic organism among the billions. They’ll need to know that on the final.
Most SEA-PHAGES participants are first-year STEM students who opt to spend double the typical weekly bio course hours in Pollenz’ lab — but the work pays off. SEA-PHAGES participants earn undergrad research credit on top of biology prereqs. They also get to name the phage they identify and register it in the GenBank — an annotated collection of DNA sequences for every known organism.
And, no pressure, but: A growing consensus within medical science is that the future of humanity could lie in the phage positioned under these students’ microscopes.
“You’re doing real-world stuff, here, contributing to a database that may have some real significance down the road,” Pollenz reminds them as their two-hour lab session winds down. “That’s powerful.”
Class is over, but the lab still hums with excitement and a sense of purpose.
Universities ignite early interest in STEM disciplines
Fewer than 40% of students who enter college intending to major in science, technology, engineering, and math fields actually complete a STEM degree.
SEA-PHAGES — an acronym for Science Education Alliance-Phage Hunters Advancing Genomics and Evolutionary Science — originated at the University of Pittsburgh in 2008 at the urging of phage researcher and biotechnology Professor Dr. Graham Hatfull.
The program is designed to meet college freshmen at the university door and to usher them into labs where the coursework is to identify a phage, analyze it, and log it into the GenBank. Because there is growing clinical evidence that phage effectively combats drug-resistant bacterial infections: every new phage SEA-PHAGES students enter in the GenBank helps push the needle forward in modern medicine.
“The idea is that in the STEM field, an emphasis on getting students a non-didactic applied learning experience that involves defined outcomes they can control and could have ownership of should be at the forefront. It’s important to get students to understand the power of research,” Pollenz says.
“The warmer we get [due to climate change], the more bacterial growth amplifies, and the more health problems we have from that perspective … the antibiotic resistance situation, as well as the number of bacteria that might impact us, is only going to increase over time. And that’s why we need real-world solutions that people are willing to invest in, right now, so that it will be easier to go from diagnosis to cure.” says Dr. Richard Pollenz, a USF research instructor
SEA-PHAGES received a $3.25 million grant from Howard Hughes Medical Institute (HHMI) in 2014, and in 2017, USF became its 11th cohort. Today, the program operates in over 100 universities across the nation.
Many of the students currently enrolled in SEA-PHAGES USF say they were introduced to the program when they attended USF-HHMI STEM Academy, a weeklong immersive experience Pollenz directs, in which incoming students connect with real-world STEM professionals.
Pollenz underscores the value of empowering students through scientific ownership. He notes that because of phage ubiquity, every student will discover a unique organism in the soil sample they take during their first semester in the program — which, for many students, is their first-ever college coursework.
“This allows each student to have 100% ownership over [their phage]. They take the sample, isolate it, and do a microscopy, so they actually get to see it — and that has a tremendous amount of power. It’s a rare thing to see what you’re working on,” Pollenz notes.
Marian Smallin, who participated in the first SEA-PHAGES cohort at USF, is now in her third year studying Cellular Molecular Biology. She’s currently preparing grad school applications.
“The [SEA-PHAGES] program itself is not easy — though I enjoy the challenge. It really helped shape my scientific identity. I’d already had the idea I wanted to be a researcher because when I was in high school, I got sick with a genetic disease. But going through the experience of being an actual researcher in my first semester — that’s what really solidified it for me. It’s why I know this is what I want to do,” says Smallin.
Phage research saves lives
In 2019, the UN World Health Organization sounded a chilling alarm on antimicrobial-resistant disease: By the year 2050, drug-resistant diseases could cause 10 million deaths, annually, and up to $100 trillion damage to the global economy. By as early as 2030, antimicrobial resistance could force up to 24 million people into extreme poverty.
“I think when Dr. Hatfull started this work, the idea we’d ever be able to really
use phages as an antibiotic felt a little bit far in the distance,” Pollenz remarks, “but the reality of what we’re dealing with, as human beings on the planet, is that bacteria are predicted to reach the critical point of being resistant to every chemical antibiotic in the next 10 to 20 years.”
Phage therapy shows demonstrable promise as an alternative to chemical antibiotics. When researchers are able to code out the bacteria-busting prowess of a specific phage, that phage can be applied to target, weaken, and even destroy deadly infection-causing bacteria such as Mycobacterium Tuberculosis, Escherichia Coli (E. Coli), Staphylococcus Aureus (MRSA), and more.
“The idea of using these particular organisms as antibiotics has always been in the literature” — Pollenz notes Nobel Prize-winning phage research during and prior to the Cold War era, and Sinclair Lewis’ 1925 novel, Arrowsmith, in which the protagonist discovers a phage that destroys bacteria during an outbreak of bubonic plague on a fictional Caribbean island — “but the reality is that only in the past three to five years have there been significant documented cases in the U.S. and the U.K. of injecting [phage] into human beings to completely remediate them from a bacteria that was otherwise going to kill them.”
Pollenz points to the recent application of phage therapy to combat bacterial infections in cystic fibrosis patients in the U.S. as an indication of the bacteria eater’s unique, but challenging, potential.
“There were four cases where people with cystic fibrosis had developed a bacterial infection that was going to kill them. Every single one had the same bacterial infection — but each one was slightly different, even though they were from the same bacterial family. That means that the phage that worked for Patient A didn’t work for Patient B, and so on — it’s very specific.”
Curing any given bacterial infection using phage therapy requires a “phage cocktail” — a patient-specific combination of phage that demonstrates lab-tested ability to attack the bacterial strain in question.
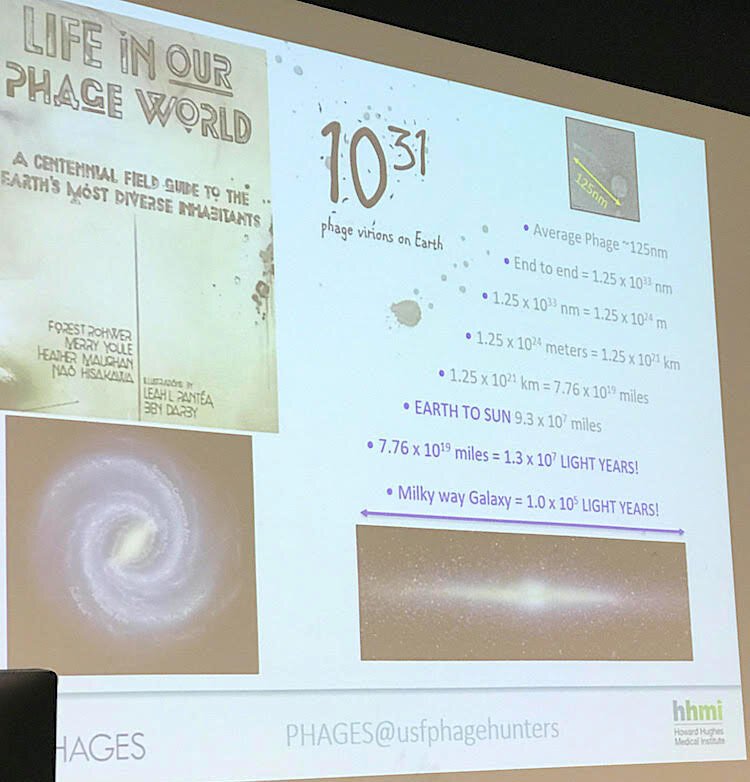
The challenge is that 1031 (that’s 10 to the 31st power) is an almost unfathomable number of phage to catalog — so many that if these microscopic organisms were lined up, they could stretch a chain from Earth to the sun, several times over.
That’s where SEA-PHAGES students’ efforts shine.
“As we continue to collect more phage into the repository, it becomes a commodity that can be mined and evaluated to see which phage are capable of infecting more pathogenic bacteria that are out there,” Pollenz says.
Pollenz suggests that phage, with more research, could even be used in the future for environmental monitoring and bioremediation.
“If phage do what we’re seeing them do to cure bacterial infections in humans, consider the possibilities: In countries that lack more sophisticated water treatment plants, for instance, phage could one day be a solution to kill bacteria in the water,” Pollenz says.
He’s confident that as more students enter science labs and develop a passion for STEM through programs like SEA-PHAGES, the brighter the future looks — in spite of the valid anxiety over climate change, and the global rise in antibiotic-resistant bacteria.
“The warmer we get, the more bacterial growth amplifies, and the more health problems we have from that perspective … the antibiotic resistance situation, as well as the number of bacteria that might impact us, is only going to increase over time — and that’s why we need real-world solutions that people are willing to invest in, right now, so that it will be easier to go from diagnosis to cure,” Pollenz says.
“I think where we go from here is forward. What these students are doing — isolating phage and putting that info into the database of possible candidates other researchers can use — is so important. You’re able to tell that student, ‘hey, the phage you named Toby could be the phage that’s used to cure someone.’ And for students who are just entering STEM fields — what a powerful hook.”
For more information, follow these links:
- SEA-PHAGES
- Dr. Richard Pollenz, USF Department of Cell Biology, Microbiology, and Molecular Biology
- Dr. Graham Hatfull, University of Pittsburgh Department of Biological Sciences
- Howard Hughes Medical Institute
- USF-HHMI STEM Academy
- HHMI Announces $60 Million Initiative to Improve Science Education for Undergraduates
- UN News April 29, 2019
- Cystic Fibrosis Patients Turn To Experimental Phage Therapy, NY Times, May 17, 2019





















